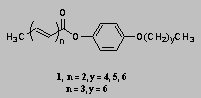9:00 HOW DO VIDEO-BASED DEMONSTRATION ASSESSMENT TASKS AFFECT PROBLEM-SOLVING PROCESSES, TEST ANXIETY, CHEMISTRY ANXIETY, ACHIEVEMENT IN GENERAL CHEMISTRY STUDENTS?
Rosalind S. Slavings* and Craig W. Bowen, University of Southern Mississippi, Hattiesburg, MS 39406
Assessment-of-learning practices can be a driving force in learning because they orient students to what is important. Our research group has focused on developing alternative techniques for assessing student learning in general chemistry. Because paper-and-pencil testing provides limited knowledge about what students know about chemical phenomena, we have developed video-based demonstrations to broaden measurement of student learning. With scores from initial and final test and chemistry anxiety instruments, the presentation will give insight on how video-based demonstration assessment tasks affect problem-solving processes, anxieties, and achievement in general chemistry students.
9:15 A DNA BINDING MODEL FOR THE ANTICANCER DRUG BIS-NAPHTHALIMIDES
Hongtao Yu*, Daekyu Sun, and Lawrence H. Hurley, Jackson State University, Jackson, MS 39217, Cancer Therapy and Research Center, San Antonio, TX 78245, and University of Texas at Austin, Austin, TX 78712
The anticancer drug bis-naphthalimides are a series of compounds with two naphthalimide moieties connected by a 6-8 carbon-nitrogen linker. The anticancer activity of these drugs is thought to be related to their ability to bis-intercalate DNA. However, the DNA binding data for these drugs is lacking. The DNA binding of these drugs has been studied by NMR, Viscometric Titration, DNA Thermomelting, and Circular Dichroism. Upon DNA-drug complex formation, the imino proton signals of DNA bases at 13 to 14 ppm shift upfield by 1-3 ppm. The upfield shift clearly indicates intercalation by the drugs into the base pairs of the duplex DNA in the DNA-drug complex. Since the upfield shift is less than 0.5 ppm for two known mono-intercalating compounds, the unusual large upfield shift also spells bis-intercalation. Viscometric titration studies confirm the drug-to-DNA base pair binding ratio to be 1:5, suggesting a bis-intercalation binding mode. Control experiments using mono-intercalating molecules further confirms the assumption. The bis-intercalation binding of drug to DNA stabilizes the duplex DNA as indicated by a higher DNA melting temperature for the drug-DNA complex than for the free DNA.
9:30 AN EFFICIENT METHOD FOR THE PRODUCTION OF 2-ADAMANTYLAMINES
Peter Ross*, Kurt A. Neidigh, Mitchell A. Avery, Sukanta Bhattacharyya, and John S. Williamson, University of Mississippi, Oxford, MS 38677
The reductive amination of aldehydes and ketones is one of the most useful methods for the preparation of amines, especially those of medicinal importance. Our interest in the development of mild and environmentally benign reagent systems has resulted in the discovery of a novel reductive amination methodology. We have used this methodology to produce a series of 2-adamantylamine derivatives in order to assess their biological properties and now wish to report the results of this study.
9:45 A NEW AND EFFICIENT METHOD FOR THE PRODUCTION OF PYRIDYLAMINE DERIVATIVES
Michael Gallotte*, Kurt A. Neidigh, Mitchell A. Avery, Sukanta Bhattacharyya, and John S. Williamson, University of Mississippi, Oxford, MS 38677
One of the most useful methods for the preparation of amines is by the reductive amination of aldehydes and ketones. Our ongoing interest in the develoment of mild and environmentally benign reagent systems has led to the discovery of a novel reductive amination methodology, using titanium(IV) isopropoxide and sodium borohydride. We have applied this methodology to the formation of a variety of pyridylamine derivatives in order to determine their biological properties and now wish to report the results of this study.
10:00 Break
10:15 X-RAY SCATTERING STUDY OF BEULAH ZAP LIGNITE SWELLED BY PYRIDINE
David L. Wertz* and Jeff L. Quin, University of Southern Mississippi. Hattiesburg, MS 39406
The objective of this research is to determine the short range structural details and the noncovalent forces which link the entanglements in coal macromolecules and to determine if (and how) liquids alter these entanglements. X-ray scattering methods, used for several years in this laboratory to study non-crystalline condensed phases, have been used to study the shortrange structuring in this lignite as well as how this structuring is altered as the lignite is swelled by the addition of a small amount of pyridine. The structural results indicate that in the lignite, the average inter-planar distance is 3.5 Å. This distance is increased to 6.6 Å by the addition of pyridine to the lignite. A molecular-model, which illustrates the alignment of adjacent poly-cyclic aromatic units in the lignite and how the pyridine molecules become intercalated between these planes, will be presented and discussed.
10:30 ENANTIORECOGNITION USING RISTOCETIN A IN A COUNTERCURRENT PROCESS IN CAPILLARY ELECTROPHORESIS
Tanya M. Oswald* and Timothy J. Ward, Millsaps College, Jackson, MS 39210
The macrocyclic antibiotic ristocetin A was evaluated as a chiral selector in a countercurrent process in capillary electrophoresis (CE). To overcome the strong absorbing nature of the chiral selector a novel approach involving the use of a coated capillary column was employed. Enantioseparations of a number of dansyl amino acids and antiinflammatories were achieved. The chiral selector was also examined as a function of separation parameters such as pH and chiral selector concentration. In this presentation the use of the macrocyclic antibiotic ristocentin A in CE, the effects of separation parameters, as well as the determination of the estimation of association constants between the chiral selector and racemic solutes by CE will be discussed. Insight into the chiral recognition mechanism can be achieved by examining the association constant between the glycopeptide and solute.
10:45 SEPARATION OF THE STEREOISOMERS OF THE MAMMALIAN ALKALOID SALSOLINOL BY HPLC
Kenneth D. McMurtrey*, Victoria G. Brower, and John G. McCoy, University of Southern Mississippi, Hattiesburg, MS 39406
Salsolinol (1-methyl-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline, SAL) has been found in the central nervous system and urine of humans and other mammals at very low levels (pmol/mL). SAL has an asymmetric carbon at position 1 and thus occurs as either the R-(+)- or S-(-)-stereoisomer. Considerable interest has been shown in the chemical literature in monitoring both the production of SAL in mammals and in determining which of the two possible stereoisomers form. Previous HPLC separations of R- and S-SAL have used primarily nonsulfated cyclodextrin together with an ion-pairing reagent. These conditions give selectivities of no more than 1.1, at most. Under conditions we have developed, the stereoisomers are separated with a selectivity of at least 1.35 with greater than baseline resolution, without the use of a separate ion-pairing reagent. The capacity factors for both stereoisomers show linear dependence with the mass of sulfated cyclodextrin in the mobile phase. This paper will also very briefly survey theories linking SAL with such human disease states as alcoholism.
11:00 INDOPHENOL DETERMINATION OF AMMONIUM ION IN THE PRESENCE OF AMINO-ACIDS. IS IT FEASIBLE?
Wedad R. Hussein*, Massomeh Khamesian, and Yolanda Underwood, Jackson State University, Jackson, MS 39217
The colorimetric indophenol method has been used for the determination of ammonia in blood serum and for urea nitrogen in urine. The method was first reported by Berthelot and later on several modifications were reported. Under the method's experimental conditions, it was found that the presence of amino acids interferes with the determination of ammonia as indophenol. Two sources of interference were identified: 1) the effect of amino acids on the pH of the solution. The method is sensitive to changes in pH, and 2) the presence of the amino group. Selective blocking of the amino group on the amino acid without affecting ammonia is one approach, though difficult, to be investigated. Amino acids were successfully removed on a cation exchange resin at a controlled pH with complete recovery of the ammonium ion in a mixture of L-aspartic acid and ammonium chloride.
11:30 Divisional Business Meeting (Petit Bois Room)
9:00 LIVING RADICAL POLYMERIZATIONS UTILIZING ORGANOMERCURIALS
Virgil T. Little*, Abul B. Kazi, and Rajive K. Khanna, University of Southern Mississippi, Hattiesburg, MS 39406
We have used diphenyl mercury as a reversible terminator in the AIBN initiated polymerization of methyl methacrylate (MMA). In living polymerizations, initiation is followed by propagation. Chain transfer and termination do not occur. The polymerization of MMA, initiated by AIBN, in the presence of diphenyl mercury results in a living polymerization with reversible termination. Diphenyl mercury forms a radical adduct with the growing polymer chain end. We believe that the mechanism of propagation involves cleavage of the carbon-mercury bond, insertion of the monomer units, and rapid reformation of the radical adduct. The polymerization of MMA follows the "livingness" equation to 60% conversion. Plots of molecular weight vs conversion, and ln(Mo/Mt) vs time show that there is no termination but some chain transfer after 60% conversion.
9:15 UV CURING PROFILES USING A NEW HIGH INTENSITY LAMP/VISIBLE PHOTOINITIATOR COMBINATION
Kip Sharp1*, Gerald Mattson1, and Roger McCartney2, 1University of Southern Mississippi, Hattiesburg, MS 39406, and 2Fusion UV Systems, Inc., Gaithersburg, MD
Ultraviolet curing of rutile TiO2 pigmented coatings is limited because TiO2 absorbs in the same region (200 nm to 380 nm) as most photoinitiators. A new high-intensity lamp source has been developed with a maximum output at 535 nm which matches a new high-efficiency, visible photoinitiator. The cure rates of both a high-gloss overprint varnish and a TiO2 pigmented coating using a standard medium pressure lamp/standard photoinitiator were compared to the new lamp/visible photoinitiator combination.
9:30 THE SILYLATION OF LESQUERELLA OIL TO ENHANCE THE CORROSION RESISTANCE OF COATINGS
C. Todd Williams*, William L. Dechent, and Shelby F. Thames, University of Southern Mississippi, Hattiesburg, MS 39406
Lesquerella oil (LO) was modified with vinyltrimethoxysilane (VTMS) to enhance the properties of LO derived coatings. Thus, an oil-modified polyester with high hydroxyl value was formed by reacting LO with glycerol and phthalic anhydride. The lesquerella oil-modified polyester (LOMP) was then reacted with VTMS via the hydroxyl functionality of the LOMP. A clear coat was formulated with the silane-modified LO polyester and a trifunctional HDI isocyanurate trimer as the crosslinker. The coating was applied to a metal panel via a drawn down bar (after treatment with a phosphate adhesion promoter) and cured at 100C for 30 minutes or at ambient temperature. In either case, the samples were allowed to equilibrate for seven days at ambient temperature prior to testing. The coatings were compared to similar coatings formulated with LOMP without silane modification and a commercial coconut oil-modified polyester (COMP) with approximately the same hydroxyl value. The VTMS modified LO polyester was superior in solvent resistance, water resistance, and pencil hardness to either of the LOMP and COMP resins.
9:45 CHLORINATED MALEINIZED GUAYULE RUBBER AS AN ADHESION PROMOTER FOR LOW ENERGY SURFACES
Michael Foster* and Shelby F. Thames, University of Southern Mississippi, Hattiesburg, MS 39406
Guayule rubber was modified by treatment with maleic anhydride and benzoyl peroxide to obtain a rubber series of varying maleic anhydride content. These materials were subsequently completely chlorinated by passing chlorine gas through a refluxing 1,2 dichloroethane solution. The rubbers were isolated by precipitation and diluted with water to 1 wt% concentration using small quantities of a cosolvent, triethylamine, and surfactant. The modified guayule rubbers were tested as adhesion promoters on polypropylene by deposition from the 1% water solution. Change in adhesive strength and surface energy of the polypropylene surface was measured by tensile pull-off and crosshatch adhesion and water contact angles, respectively. These results were compared to two commercial chlorinated polyolefins (CPOs), one each of solventborne and waterborne. The adhesive strength increased as a function of decreasing maleic acid content while in this same series water dispersibility decreased. A variety of maleic anhydride to chlorine ratios failed to improve adhesion compared to solventborne CPO. However, maleinized chlorinated rubber treatment gave improved adhesion compared to the waterborne CPO system.
10:00 Break
10:15 WHY DO WE STUDY CHEMICAL REACTIONS IN MICROGRAVITY?
John A. Pojman, University of Southern Mississippi, Hattiesburg, MS 39406
NASA funds a great deal of research on chemical processes under weightless conditions. Why? It is rarely to develop processing in space because of the high cost. Instead it is usually for one of two reasons. If buoyancy-driven convection interferes with the quality of a material, then producing a benchmark material to use as a standard for ground-based processing is valid. The second reason is the test the role specific prediction of the role of buoyancy-driven and surface-tension induced convection in chemical reactions. We will consider examples from the PI's research on frontal polymerization in microgravity, including a sounding rocket flight and several KC-135 flights.
10:30 AN INVESTIGATION OF ORGANIC DYE GRADIENT MATERIALS
Jim Owens* and John A. Pojman, University of Southern Mississippi, Hattiesburg, MS 39406-5043
Gradient Refractive INdex(GRIN) materials are being perfected for use in high-bandwidth communications, optical limiters, and specialized lenses. We propose that GRIN materials may be produced by introducing an organic dye to the system such that it will disperse through a polymer to form a dye gradient. Such systems have been proposed for use in optical limitation and amplification of light. A proposed system for polymerization is via Isothermal Frontal Polymerization (IFP). In IFP, a polymer seed is submerged in a monomer/thermal initiator/inhibitor solution. IFP initiates inside the seed and propogates through the solution. The introduction of an organic dye to the seed or solution will produce an optical dye gradient.
10:45 POLYMERIZATION OF ACRYLONITRILE COUPLED TO AN OSCILLATING REACTION
Randy Washington*, Gauri P. Misra, and John A. Pojman, University of Southern Mississippi, Hattiesburg, MS 39406
Polymerization of acrylonitrile has been shown to occur periodically with oscillations in a batch reactor. Two radicals that are formed in the Belousov-Zhabotinsky system play a major role. The malonyl radical initiates polymerization, while the bromine dioxide radical is suspected of terminating the polymer chain. Periodic polymerization has also been found to exist in other organic system such as lactic acid, citric acid, and oxalic acid as well as the Mn(II) oscillating reaction. A variable, alpha, defined as the ratio of malonic acid to bromate concentration, relates the dynamics of the reaction and the chain length of the polymers. Thus, the molecular weight distributions as a function of alpha is investigated. Temperature profiles, light transmittance (monitoring polymer precipitation), and platinum potential data indicate that the dynamics of the reaction affects the kinetics of the polymerization process. Simulations of the model for polymerization coupled to oscillations agree fairly well suggesting that the model is able to predict the general behavior.
11:00 THE EFFECT OF INITIAL COMPOSITION ON FRONT VELOCITY IN BINARY FRONTAL POLYMERIZATION AND FRONTAL COPOLYMERIZATION: COMPARISON OF THEORY TO EXPERIMENT
Jerry Griffith*, Jim Owens, and John A. Pojman, University of Southern Mississippi, Hattiesburg, MS 39406
Binary frontal polymerization is a propagating localized reaction of two noninterfering polymerization systems, e.g., cationically-cured epoxy and free-radical cured diacrylates. The velocity always exhibits a minimum as a function of the relative fraction of the two components, which means the fronts a solution of the two components will always support a slower front than either system alone. However, frontal copolymerization in which two monomers can react with each other through a free-radical mechanism can show a maximum or minimum, depending on the values of the reactivity ratios. We have developed simple analytical conditions that predict these phenomena, in excellent agreement with experimental results.
11:30 Divisional Business Meeting (Petit Bois Room)
1:30 Divisional Poster Session
DNA PHOTOCLEAVAGE BY ANTICANCER/ANTIBACTERIAL DRUG QUINOBENZOXAZINES
Hongtao Yu1*, Yan Kwok2, Sean M. Kerwin2, and Lawrence H. Hurley2, 1Jackson State University, Jackson, MS 39217, and 2University of Texas at Austin, Austin, TX 78712
The anticancer/antibacterial quinobenzoxazines decompose upon light irradiation. The photodecomposition is greatly accelerated by the formation of drug-DNA complexes in the presence of Mg2+ and, as a result, the duplex DNA is photocleaved. The photocleavage mechanism and photocleavage products are examined using plasmid and oligomer DNA photocleavage assays in the presence of free radical scavengers, excited-state quenchers, transition metal ions, and oxygen or argon. A free radical DNA cleavage mechanism is proposed: in the excited singlet state, the quinobenzoxazine transfers an electron to an electron acceptor, oxygen or a DNA base, and the oxygen or DNA base radical thus formed causes DNA strand breakage. The breakage is mostly single strand breaks on duplex DNA. Two to three DNA photocleavage products are identified using sequencing gel-electrophoresis: two frank strand break products and an abasic product. Active oxygen species seems to be partially involved in the photocleavage of DNA.
SEARCHES ON THE POTENTIAL ENERGY SURFACES OF BNH2, AlNH2, BPH2, AND AlPH2
Dee McMillan1U*, Brian Lamb1, David H. Magers1 and Jerzy Leszczynski2, 1Mississippi College, Clinton, MS 39058, and 2Jackson State University, Jackson, MS 39217
Optimum geometries are computed at both the SCF level and the level
of second-order perturbation theory for several conformational isomers
on the potential energy hypersurfaces of BNH2, AlNH2,
BPH2, and AlPH2, including linear structures, methylenecarbene-like
structures, and mono-bridged and double-bridged structures. Comparisons
with isoelectronic systems such as C2H2, CSiH2,
Si2H2, and CGeH2 are also discussed. In
addition, harmonic vibrational frequencies are computed to characterize
all structures considered as local minima or transition states. Infrared
intensities within the double harmonic approximation are also determined.
Two basis sets, both of triple-zeta quality on valence electrons, are employed
for all computations. One set contains one d polarization function on all
heavy atoms and one p function on all hydrogens; the other includes
two d and one f polarization function on all heavy atoms
and two p functions and one d polarization function on each
hydrogen. Previous investigations of ours indicate that large basis sets
such as those employed in this study can in part compensate for the lack
of a more advanced treatment of electron-correlation. We gratefully acknowledge
support from NSF EPSCoR (OSR-9452857).
WAVE PACKET DYNAMICS OF LOCAL MODE WATER
Jennifer K. Tonos*, Mary K. Zaremba, and Joseph A. Bentley, Delta State University, Cleveland, MS 38733
We report 3D wave packet dynamics for water in the ground electronic state. The Radau coordinate system is used. The discrete variable representation (DVR) [Z. Bacic and J.C. Light, Annu. Rev. Phys. Chem. 40, 469 (1989)] is used as a basis for all coordinates. The initial state is constructed in such a way as to mimic water in a "pure" local mode state. The wave packet propagation is achieved by using the Chebyshev propagator [H. Tal-Ezer, and R. Kosloff, J. Chem. Phys. 81, 3967 (1984)]. A new global potential energy surface for water [H. Partridge and D.W. Schwenke, J. Chem. Phys. 106, 4618 (1997)] is used in the calculation. Methodology is presented, along with some preliminary results.
HIGH VIBRATIONAL ENERGIES OF WATER
Gabrielle Balam*, and Joseph A. Bentley, Delta State University, Cleveland, MS 38733
We report the accurate large scale calculation of 3D vibrational eigenstates of the ground electronic state of water. The Radau coordinate system, originally developed over a century ago for the study of planetary systems, is used. The discrete variable representation (DVR) [Z. Bacic and J.C. Light, Annu. Rev. Phys. Chem. 40, 469 (1989)] is used as a basis in the Hamiltonian matrix for all coordinates. The eigenvalues are obtained by using the Implicitly Restarted Arnoldi Method (IRAM). This is part of a recently developed numerical package (ARPACK) designed to solve large scale eigenvalue problems. The energies are calculated using a new global potential energy surface for water [H. Partridge and D.W. Schwenke, J. Chem. Phys. 106, 4618 (1997)].
DYNAMIC NMR AND QUANTUM MECHANICAL STUDIES ON BARRIER TO INTERNAL ROTATION OF BIURET
Rhonda Odom*, Ali Jabalameli, Andrzej Nowek, and Richard Sullivan, Jackson State University, Jackson, MS 39217
The temperature dependent H1NMR spectra of biuret in a mixture
of 80% CDCI3 and 20% DMSO-D6 were recorded. Three
barriers to internal rotation about the C-N bonds were determined (AG==14.01,
11.48, 11.18 kcal/mole).The results indicated that the intramolecular hydrogen
bondings(C=O...... HN) have a significant effect on these rotational energies.
The trans-cis form was found to be the predominant conformation for this
compound. In addition to the NMR measurements a high level ab initio studies
were performed at the second-order Möller-Plesset perturbation theory
(MP2) level of theory using the extended triple-zeta Pople's 6-31 lG(d,p)
basis set. The geometry optimizations revealed three minimum energy structures
of which the trans-cis form is more stable. The transition state structure
connected to each possible pair of biuret isomers were located and the
calculated barrier heights were compared to the experimental data.
QUANTUM MECHANICAL STUDY ON MOLECULAR STRUCTURE AND STABILITY OF THE HYDROXYUREA-AMMONIA COMPLEX IN GAS PHASE AND IN SOLVENT
Akilah Shelby*, Teri Lynn Robinson, Ali Jabalameli, Andrzej Nowek, Ramaiyer Venkatraman, and Richard Sullivan, Jackson State University, Jackson, MS 39217
The hydroxyurea-ammonia complex was studied by high level ab initio
quantum mechanical methods. The equilibrium hydrogen bonded structure,
interaction energy and its components, harmonic vibrational frequencies
of the complex in gas phase were calculated at the second-order Möller-Plesset
perturbation theory (MP2) level using the extended triple-zeta Pople's
6-311++G(df,pd) basis set. The proton transfer pathway was also investigated
at the same level of theory. The influence of water as a solvent on molecular
structure and stability of the studied complex was determined adopting
the self-consistent reaction field (SCRF) theory using self-consistent
isodensity polarized continuum model (SCI-PCM).
QUADRUPOLE COUPLING CONSTANT CALCULATION OF BROMINATED AROMATIC COMPOUNDS USING GAMESS
Akilah Shelby1*, Richard Sullivan1, Chris Harwell2, and Randall Hall2, 1Jackson State University, Jackson, MS 39217, and 2Louisiana State University, Baton Rouge, LA
The objective of this research is to calculate the Quadrupole Coupling Constants for brominated aromatic compounds. All calculations used the GAMESS program employing ab initio self consistent field methods and multiple basis sets. Specifically, the program is utilized calculate the electric field gradient at the bromine nuclei in each molecule whose primary axis is then used in the calculation of the QCC. Trends were reported for kite QCC as a function of Br-Br distance as well as effects of increasing the number of bromine atoms on the benzene ring. These calculations have direct application in the use of Nuclear Quadrupole Resonance Spectroscopy in the detection of aromatic bromines used as flame retardants in polymers such as television cabinets.
MICROBIAL ECOLOGY OF SELENIUM DAMAGED ENVIRONMENTS PROJECT
Teri Lynn Robinson1*, Richard Sullivan1, Xinghua Zeng2, Terrance Leighton2, and E.O. Lawrence3, 1Jackson State University, Jackson, MS 39217, 2University of California, Berkeley, CA, and 3Berkeley National Laboratory, Berkeley, CA
Selenium pollution is one of the most widely encountered examples of
metal hazards to the environment in the Western United States. Toxic species
of selenium are pollutants found in groundwater, smelting effluents, agricultural
and municipal waste water, waste disposal sites, power plant cooling reservoirs,
and oil refinery waste streams. Due to agricultural sources of selenium,
our research objective focuses on the substantial selenium burden found
in drainage canals located in the California Central Valley. Ecological
studies of selenium damaged environments has entailed the isolation of
strains from ten sites and the preservation of each strain by a frozen
stock method. Each isolated strain was further characterized and classified.
THE SYNTHESIS AND CHARACTERIZATION OF BRIDGING PHOSPHINE COMPLEXES
Samuel J. Lewis1*, Laura T. Smith2, and Judith L. Eglin2, 1Mississippi University for Women, Columbus, MS 39701, and 2Mississippi State University, Mississippi State, MS 39762
The compound (Bu4N)2Re2Cl4
is an excellent starting material for synthesizing bridging phosphine complexes
of the type Re2Cl4(L-L)2, where L-L =
dcypm [bis(dicyclohexyl-phosphino)methane] or dcype [bis(dicyclohexyl-phosphino)ethane].
Reactions of (Bu4N)2Re2Cl8
and (Bu4N)2Re2Cl6P2
with dcype and dcypm, respectively, forming compounds with dcype ligands
bridging the dirheniurn metal centers of the (Bu4N)2Re2CI8
and dcypm ligands bridging the dirhenium metal centers of the (Bu4N)2Re2Cl6P2
were accomplished. The compounds were characterized using 3 lPNMR
and ultraviolet-visible spectroscopy. Synthesis and characterization will
be discussed.
PREPARATION AND CHARACTERIZATION OF DEOXYEPHEDRINIUM MANDELATES
Marcus Harris* and Edward J. Valente, Mississippi College, Clinton, MS 39058
Solubility in 95% ethanol generally increases considerably between comparable
ephedrinium mandelates and pseudoephedrinium mandelates. Since these diastereomeric
salt systems differ only at the configuration of the benzylic hydroxyl
of the cations, and since intramolecular H-bonding is a consistent feature
of the more soluble phases, we sought to explore the structures and properties
of the corresponding system lacking the hydroxyl group in the cation. As
a beginning study, we have prepared and investigated the diastereomeric
discriminatory behaviour of binary salts between (substituted) mandelate
salts and (-)-deoxyephedrine. We have also begun a comparison of their
structures with related salts having benzylic hydroxy groups in the cations.
We acknowledge instrumental support from NSF (DUE#8950384, #9250769), NIH
(GM42198) and the Office of Naval Research.
PSEUDOACID AMINO ACIDS? FORMATION AND STABILITY OF 2-AMINO-5-KETOCARBOXYLIC ACIDS
Lefferage Robbins* and Edward J. Valente Mississippi College, Clinton, MS 39058
Among the twenty usual amino acids found in mammalian biochemistry,
examples with side chain aldehyde or keto functions are unknown. The closest
case is 2-amino-4-oxobutanoic acid which, in the correct environment, spontaneously
cyclizes prior to enzymatic oxidation to form proline. A case can be made
that 4- and 5-oxo-2-aminocarboxylates are unstable with respect to conversion
either toward the intramolecular cyclic aminals or the cyclic pseudoacids.
The former may be a relative of the Maillard reaction, otherwise known
as nonenzymatic browning. To explore this area of chemistry, we have prepared
derivatives of suitably protected 5-oxo-2-amino acids. The isolation and
stability of the parent compounds will be presented along with procedures
for preparation and stabilization of the derivatives. We acknowledge instrumental
support from NSF (DUE#8950384, #9250769), NIH (GM42198) and the Office
of Naval Research.
SYNTHESIS AND STRUCTURE OF DERIVATIVES OF o-CARBOXYPHENYLACETALDEHYDE
Brian McFarland* and Edward J. Valente, Mississippi College, Clinton, MS 39058
The 5-oxoacid o-carboxyphenylacetaldehyde exists entirely in the cyclic pyranoid pseudoacid form in the solid state and in solutions of nonpolar solvents. We have begun to investigate the derivatives of this pseudoacid system which can be prepared through the pseudoacid chloride. The pseudoacid itself is prepared by a new method beginning from 1-indanone, concluding with the traditional periodic acid oxidative cleavage of 2-hydroxy-1-indanone. The stable cyclic pseudoacid chloride may be converted to other pyranoid pseudoacid derivatives such as the analogous amides and and esters. For example, with the primary amine propylamine, the N-propyl amide of the pseudoacid forms; with the secondary amine morpholine, the N-morpholinyl amide forms. The spectroscopy and solid state structures will be presented, and these have been compared with other normal and pseudo-derivatives of organic acids.
We acknowledge instrumental support from NSF (DUE#8950384, #9250769), NIH (GM42198) and the Office of Naval Research.
STRUCTURE OF 1-CYCLOPENTANO-3-THIOSEMICARBAZONE AND 1-CYCLOPENTANO-4-ETHYLTHIOSEMICARBAZONE
Akilah Shelby1, Christie Davis1*, Ramaiyer Venkatraman1, Jeffrey D. Zubkowski1, and Edward J. Valente2, 1Jackson State University, MS 39217, and 2Mississippi College, Jackson, MS 39058
1-Cyclopentano-3-thiosemicarbazone and the corresponding ethyl derivative was synthesized and single crystal X-ray diffraction studies were carried out on the two compounds. Crystal data reveals that these two belongs to tetragonal class with P41 or P43 group and has the syn, E,Z conformation. Both form N-H..N intramolecular hydrogen bonds. The solid state structures are consistent with their infrared and proton magnetic resonance spectra.
SYNTHESIS, CHARACTERIZATION AND FORMATION CONSTANTS OF THE COMPLEXES OF Cu(II), Co(II), AND Fe(III) WITH DIHYDROXY ACETOPHENONE 4N-SUBSTITUTED THIOSEMICARBAZONES
Kristie Davis*, Penny Lindsey, and Ramaiyer Venkatraman, Jackson State University, Jackson, MS 39217
Binuclear Cu(II), Ni(II) and Fe(III) complexes with 2,4-dihydroxy acetophenone 4N-substituted thiosemicarbazones have been synthesized and characterized. IR, W, 1HNMR spectra of the complexes, as well as that of the thiosemicarbazones, have been obtained. Stepwise formation constants of the metal complexes were obtained in presence of 0.1 M sodium chloride in DMF solution at 25°C potentiometrically employing methods of Bjerrum and Irving and Rossotti. Magnetic and conductance data for the complexes are also determined. Based on the information, the nature and structure of the metal complexes were determined.
SYNTHESIS OF TRANS-DIBENZYL-2,2'-DIPYRIDYLDIOXOMOLYBDENUM (VI) COMPLEX AND ITS REACTION WITH LITHIUM DIISOPROPYLAMIDE
Ken S. Lee and Shon-Keith Booker*, Jackson State University, Jackson, MS 39217
Trans-Dibenzyl-2,2'-dipyridyldioxomolybdenum (VI) (4) was synthesized by the reaction of benzylmagnesiumchloride with dibromodioxo-2,2'dipyridylmolybdenum (VI) (3) as described in the literature. Dibromodioxo-2,2'dipyridylmolybdenum (VI) (3) was also prepared from molybdenum hexacarbonyl (1) in two steps as below and the overall yield of reaction was 32.5%. 1H NMR and IR were used to interpret the structure of complexes. The 1H NMR spectrum shows that the complex (4) decomposes to produce benzaldehyde in aerobic condition as the literature described. The reaction of the molybdenum complex (4) with lithium diisopropylamide (LDA) at low temperature and its chemistry will be discussed in detail.
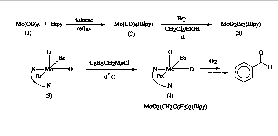
LIGHT INDUCED PHOTODEGRADATION OF PHENOLPHTHALEIN CATALYZED BY TiO2
Hilda J. Wells* and Ken S. Lee, Hinds Community College, Jackson, MS 39213, and Jackson State University, Jackson, MS 39217
Photodegradation reaction of phenolphthalein in basic aqueous solution
at room temperature was carried out by measuring the UV absorption at 550
nm during irradiation. Titanium oxide was used as a catalyst for photodegradation.
The reaction follows a first order kinetics for phenolphthalein. Its half
life of phenolphthalein is 47.6 min. The rate law and the structure of
possible end product of degradation will be discussed in detail.
9:00 DETERMINATION OF THE MAGNETIC SUSCEPTIBILITIES OF VARIOUS COPPER (II) LEVULINATE COMPOUNDS
Tina L. Steele*, Kiwana T. Johnson, and Jeffrey D. Zubkowski, Jackson State University, Jackson, MS 39217
The d-metals have come into great interest of late in the area of inorganic chemistry. These metals, when combined with different ligands can form a variety of complexes. The d-metal of interest is that of copper (II). The complexes studied are those formed with levulinic acid. The complexes of the following copper levulinate compounds will be presented: Cu2(lev)4, Cu2(lev)4(OC5H4NH), and Cu2(lev)4(4-CNpy). Magnetic susceptibilities, behavior of each of the compounds, magnetic moments, and other related information for each compound will be presented. The methods by which the information about these compounds was attained will also be presented.
9:15 SEARCHES ON THE POTENTIAL ENERGY SURFACES OF NCH2, NSiH2, NGeH2, PCH2, PSiH2 AND PGeH2 AND THEIR CORRESPONDING CATIONS
Sharon M. Garner1U*, Terri Darby1U, David H. Magers1, and Jerzy Leszczynski2, 1Mississippi College, Clinton, MS 39058, and 2Jackson State University, Jackson, MS 39217
Optimum geometries are computed at both the SCF level and the level of second-order perturbation theory for several conformational isomers on the potential energy hypersurfaces of NCH2, NSiH2, NGeH2, PCH2, PSiH2, and PGeH2, including linear structures, methylenecarbenelike structures, and mono-bridged and double-bridged structures. The corresponding monocations are also investigated to note similarities and differences between their optimum structures and those of the neutral systems. Comparisons of the cations with neutral isoelectronic systems such as C2H2, CSiH2, Si2H2, and CGeH2 are also discussed. In addition, harmonic vibrational frequencies are computed to characterize all structures considered as local minima or transition states. Infrared intensities within the double harmonic approximation are also determined. Two basis sets, both of triple-zeta quality on valence electrons, are employed for all computations. One set contains one d polarization function on all heavy atoms and one p function on all hydrogens; the other includes two d and one f polarization function on all heavy atoms and two p functions and one d polarization function on each hydrogen. Previous investigations of ours indicate that large basis sets such as those employed in this study can in part compensate for the lack of a more advanced treatment of electron-correlation. We gratefully acknowledge support from NSF EPSCoR (OSR-9452857).
9:30 THEORETICAL INVESTIGATIONS OF THE STABILITY AND RING STRAIN OF OXAZIRIDINE
Lee L. TurnerU*, Lee LewisU, and David H. Magers, Mississippi College, Clinton, MS 39058
Oxaziridine, a ring isomer of ONCH3, has never been isolated to our knowledge, but derivatives of oxaziridine are widely found in the organic chemistry literature. Equilibrium geometries have been optimized and harmonic vibrational frequencies have been calculated at both the SCF level and the level of second-order perturbation theory using three split-valence basis sets: 6-31 lG(d,p), 6-31 lG(2df,2p), and 6-31 lG(2df,2pd). Previous investigations of ours indicate that large basis sets such as these can in part compensate for the lack of a more advanced treatment of electron-correlation. These frequency determinations indicate that oxaziridine is a local minimum on the ONCH3 potential energy hypersurface. Optimum geometries and corresponding energies are also computed for formamide, nitrosomethane (ONCH3), and hydroximinomethane (HONCH2) for comparison purposes. Finally, the ring strain in oxaziridine is estimated using the isogyric, isodesmic, and ho: nodesmotic models. These results are compared to other small ring isoelectronic species like N3H3, cyclopropane, and ethylene oxide. This research was supported by an award from Research Corporation.
9:45 SOLUTION ION CHEMISTRY OF CEMENT COMPONENTS: KINETICS OF DISSOLUTION AND PHASE TRANSFORMATION
C.A. Weiss, Jr.*, S.L. Larson, and T.S. Poole, US Army Engineer Waterways Experiment Station, Vicksburg, MS 39180
In order to understand the reactions associated with hardening of cement, time-dependent solubilization of a number of components common to cementitious systems were performed. The concentration of calcium and sulfate ions in solution was measured using high performance ion chromatography. The samples of the three basic components of portland cement, calcium sulfates, calcium silicates, and calcium aluminates, show varied levels of calcium and sulfate ions in solution. The sulfate containing components of cementitious materials all show small sulfate concentrations in solution over time. This can be explained by dissolution of CaSO4 in the absence of any competing, reactions which remove the ions from solution. Sulphate levels in solutions above calcium silicate and calcium aluminate cement components contain low levels of sulfate ion. Cement mixtures contain an appreciable amount of calcium sulfates, calcium silicates, and calcium aluminates; however, levels of sulfate ion in solution are observed to be low in solutions above these mixtures as well. This suggests that either a competing reaction exists that removes sulfate from solution on a time scale similar to that of dissolution or that a protective covering is quickly forming around the particles that is inhibiting the interaction between the water and the crystalline CaSO4. Conduction calorimetry and x-ray diffractometry is utilized in order to explain the role of complex mixtures of calcium sulfates, calcium silicates, and calcium aluminates in the hardening of cement.
10:00 ADSORPTION OF ORGANIC CATIONS BY SOIL COMPONENTS EXAMINED USING COMPUTATIONAL CHEMISTRY
S.L. Larson*, J.W. Adams, and C.A. Weiss, Jr., US Army Engineer Waterways Experiment Station, Vicksburg, MS 39180
Clay mineralogists have observed that organic and inorganic cationic species present in the interlayer of expandable clays cause variable expansion of individual aluminosilicate layers [MacEwan, D.M.C.,and Wilson, M.J., 1980]. In the case of inorganic cations, these distances are defined by the size of the cation and the volume of the hydration sphere surrounding the cation. Organic cations, because of the variations of the molecular geometry and orientation within the clay interlayer, can produce a range of interlayer distances depending on the cation orientation and the shape of the cationic species. A number of hydrated organic cations were modeled using a number of PC-based molecular modeling techniques and the results were compared to distances determined using X-ray diffractometry for organo-clays prepared using the same organic cations. The result explain the mechanism of the range of distances observed for clays containing organic cations. Dependencies of the interlayer spacing were noted between the molecular geometry of the cations studied, the amount of organic cation sequestered by the clay, and the cation exchange capacity of the expandable clays studied. The information provided is used to explain the sequestration of organic cations of environmental interest (cationic pesticides, herbicides, and explosives) in soils containing expandable clays. MacEwan, D.M.C., and Wilson, M.J., (1980). "Interlayer and intercalation complexes of clay minerals." Crystal structures of clay minerals and their X-ray identification. G. Brindley and G. Brown, ed., Mineralogical Society, 203.
10:15 Break
10:30 ORIENTATION DEPENDENCE OF THE FRONT VELOCITY OF DESCENDING THERMAL FRONTS
Archie Nichols1*, John A. Pojman1, Alexander Segal2, and Vital Volpert3, 1University of Southern Mississippi, Hattiesburg, MS 39406, 2Institute of Fine Mechanics and Optics, St. Petersburg, Russia, and 3Université Lyon I, Lyon, France
A descending thermal front of a reaction that produces a solid product from a liquid reactant can be affected by gravity through the convection produced under the front if the tube is not vertical. We studied the frontal polymerization of acrylamide/bisacrylamide in DMSO as a function of the tube angle. The closer the tube was to horizontal, the faster was the front. That the convection and not the surface area of the front was cause was determined in a KC-135 microgravity flight. The effect of the initial viscosity and the intrinsic front velocity were studied and compared to numerical simulations.
10:45 PERIOD-DOUBLING IN PROPAGATING FRONTS OF 1,6-HEXANDIOL DIACRYLATE POLYMERIZATION
Jonathan Masere* and John A. Pojman, University of Southern Mississippi, Hattiesburg, MS 39406-5043
Free radical polymerization reactions show thermal autocatalysis. When this autocatalysis is coupled to the kinetics of free radical polymerization reactions in homogeneous and unstirred reactors, frontal polymerization is afforded. Frontal polymerization is a process in which the reaction is initiated at one point of the reactor and the heat generated diffuses into the adjacent zone containing fresh reactants to initiate further polymerization. This process can be self-sustaining under appropriate conditions. As an industrial method for preparing polymeric materials, frontal polymerization offers two major advantages over the conventional methods, viz.; the heat produced by the reaction is utilized to effect further polymerization and there is no risk of thermal runaway as would be in the case with conventional methods. Using this method, specialty polymeric materials have been produced. To enhance the quality of these materials, it may be necessary to use multifunctional acrylate monomers which impart mechanical strength due to cross-linking. However, these acrylates tend to gel at very low conversions and Hoyle and co-workers have shown that there is a corresponding increase in the activation energy. This increase can culminate in non-planar polymerization fronts that adversely affect the quality of the resultant products. We will present results of our findings that pertain to some of the conditions that can cause the emergence of nonplanar fronts.
11:00 AN EXPERIMENTAL INVESTIGATION OF THE MECHANISM BEHIND ISOTHERMAL FRONTAL POLYMERIZATION
L. Lee Lewis* and John A. Pojman, University of Southern Mississippi, Hattiesburg, MS 39406-5043
Isothermal Frontal Polymerization (IFP) is a mode of converting monomer into polymer via a localized propagating reaction zone and occurs when a polymer seed, i.e. a small piece of polymer, is placed in contact with a solution including its monomer and a thermal initiator. The primary industrial use of isothermal fronts is in the production of gradient optical materials such as Gradient Refractive INdex (GRIN) Materials. While GRIN materials are in use, there is not a clear understanding of the factors that affect the process of IFP. Our efforts to examine these factors found that the average distance of front travel is 1.0 ± 0.3 cm and that the time of travel is approximately 24 h. It was also found that the front is highly dependent on the combination of monomer, initiator, and inhibitor used.
11:15 STIMULI-RESPONSIVE ZWITTERIONIC CYCLOCOPOLYMERS: SYNTHESIS AND SOLUTION BEHAVIOR OF A NOVEL CLASS OF POLYAMPHOLYTES
R. Scott Armentrout*, Michael F. Richardson, and Charles L. McCormick, University of Southern Mississippi, Hattiesburg, MS 39406
A novel zwitterionic monomer, 3-(N,N-diallyl-N-methyl ammonio) propane sulfonate (DAMAPS) was synthesized from the starting material, N,N-diallyl-N-methylamine (DAMA). Cyclocopolymers of N,N-diallyl-N,N-dimethylammonium chloride (DADMAC) and DAMAPS and cyclocopolymers of DAMA and DAMAPS were prepared by free radical copolymerization in 0.5 M NaCl solution. Copolymer compositions were determined by gated decoupled and DEPT135 13C n.m.r. Incorporation of the zwitterionic mer unit was not found to alter the formation of the five-membered ring commonly seen in DADMAC and DAMA homopolymers. Solution behavior and chain dimensions of these novel polyampholytes were determined as a function of pH, ionic strength and mol% incorporation of the zwitterionic mer unit by viscometry, dynamic light scattering, and fluorescence spectroscopy.
11:30 SYNTHESIS AND CHARACTERIZATION OF MONOMERIC AND POLYMERIC VESICLES FORMED BY THE NOVEL MONOMER N,N-DIALLYL-N,N-DIDECYLAMMONIUM CHLORIDE
Michael F. Richardson*, Yuxin Hu, Monica Tisack, and Charles L. McCormick, University of Southern Mississippi, Hattiesburg, MS 39406
The novel amphiphilic monomer N,N-diallyl-N,N-didecylammonium chloride has been synthesized. Photopolymerization of the monomer with N,N-diallyl-N,N-dimethylammonium chloride yields water soluble copolymers. Characterization of the monomer in aqueous solution suggests that the monomer aggregates into well defined structures above a critical concentration. Dynamic light scattering and atomic force microscopy indicate the size of the aggregates to be approximately 350 nm in diameter. Fluorescence measurements provide further evidence that the microdomains of the monomer aggregates are ordered and moderately polar in nature. These dimensions and properties are attributed to large vesicular structures of the monomer. Surface tension, dynamic light scattering and fluorescence measurements have been performed with the monomer and added surfactants. The results of these studies show that the didecyl monomer associates well with surfactants across a range of concentrations. An understanding of the association tendencies of the didecyl monomer in solution provides a firm basis for analysis of the properties of polymers and copolymers incorporating the N,N-diallyl-N,N-didecylammonium chloride monomer.
1:30 POLYMER CHAINS AT THE SURFACE: ON-OFF-LATTICE HYBRID SIMULATION APPROACH
Grace M. Foo, National University of Singapore, Singapore, Republic of Singapore, and R.B. Pandey*, University of Southern Mississippi, Hattiesburg, MS 39406
A hybrid computer simulation method is proposed to study the static and dynamic properties of polymer chains. We use both, continuum and discrete lattice methods to implement the procedure. With the discrete lattice method, we accelerate the polymer chains leading to mixing at large scale followed by continuum method to relax and explore the local modes. As an example we consider polymer chains on a two dimensional surface with various polymer interactions and study their conformations and dynamics along with their radial distribution functions as a function of temperature and polymer concentration. Effects of quenched barriers may also be discussed. This work is supported in part by a NSF-EPSCoR grant.
1:45 ELASTOMERIC NEMATIC POLYESTERS-- TOWARDS AUXETIC MATERIALS
Chad J. Booth* and Anselm C. Griffin, University of Southern Mississippi, Hattiesburg, MS 39406
This research is designed to use elastomeric nematic polyesters in the development of auxetic materials. Auxetic materials are those that exhibit a negative Poisson's ratio (lateral expansion upon stretching). Currently there are some materials that exhibit this behavior but these are based on a macroscopic design. The materials developed here will be designed to show this effect based on a microscopic design. The polymers currently being studied utilize elastomeric LC polyesters containing both terminally attached as well as laterally attached mesogenic groups or rods. Changing the feed ratios of the monomers used in the polymerization can control both the level of crosslinking and the amount of incorporation of transverse rods. This study will focus on the effect of crosslinking as well as the introduction of laterally attached rods on the LC behavior of the polymers. Information obtained to date shows that there is retention on the LC phase with addition of both crosslinker and transverse rod. There is however a loss of crystallinity at higher levels of crosslinking. Also seen is an increase in the clearing temperature (TN-I) and a decrease in the glass transition temperature (Tg) as crosslinking is increased (shown by DSC). We wish to thank the NSF (DMR-9420843) for support of this work and for the acquisition of thermal instrumentation facilities through (DMR-9512506).
2:00 LIQUID CRYSTALLINE POLYMERS AS POTENTIAL AUXETIC MATERIALS
Puwei Liu*, Chaobin He, and Anselm C. Griffin, University of Southern Mississippi, Hattiesburg, MS, 39406
Auxetic materials have been the subject of much recent inquiry. The phenomenon involves the lateral expansion of materials when stretched. There is to date no molecularly designed auxetic material. Our research focuses on a molecular engineering approach to the design and synthesis of polymeric materials which can exhibit this unusual mechanical response. We will report on the properties of some main chain liquid crystalline polymers having a transversely-attached rod. These nematic polymers have been examined by x-ray scattering and have been shown to exhibit an increase in the interchain distance when formed into fibers (stretched state). This result validates our design concept for auxetic polymers. This work was supported by the AFOSR through grant F-49620-94-1-0454.
2:15 EFFECT OF MOLECULAR STRUCTURE ON COMPLEX FORMATION IN SOME CARBOXYLIC ACID/PYRIDINE ADDUCTS
Clemon Terrell* and Anselm C. Griffin, University of Southern Mississippi, Hattiesburg, MS 39406
Carboxylic acids and pyridines can form 1:1 molecular adduct via hydrogen bonding between the -COOH hydrogen and the lone pair of electrons on the pyridine nitrogen. We have used this strongly directional hydrogen bond to construct new liquid crystalline compounds including polymers. In this study we report the design, synthesis and characterization of several carboxylic diacid/bispyridyl complexes which can be used to examine the effect of lateral substituents on the crystallinity and the mesophase stability of our polymers.
2:30 Break
2:45 PHOTOCHEMICALLY REACTIVE SIDE-CHAIN LIQUID CRYSTALLINE POLYMERS CONTAINING THE 4,4'-DIALKOXYSTILBENE CHROMOPHORE
Shivkumar Mahadevan and David Creed*, University of Southern Mississippi, Hattiesburg, MS 39406
The photochemistry of liquid crystalline polymers has several possible technological applications and is of fundamental interest because liquid crystalline materials are partly ordered yet retain the ability to flow. We report the synthesis of four new side-chain substituted polymers with a fluorescent, photochemically reactive 4,4'-dialkoxystilbene chromophore attached by a flexible C6 or C11 'spacer' to either methacrylate or styrene-based type main chains. For example, 4-pentyloxybenzyl chloride was coupled by an Arbuzov reaction with triethylphosphite to obtain the corresponding phosphonate, the anion of which, generated using sodium hydride in dimethyl formamide, afforded the trans-stilbene by Horner-Emmons reaction with the tetrahydropyranyl ether of 4-(6'-hydroxyhexyloxy)benzaldehyde. Deprotection of the resultant trans-stilbene with dilute acid afforded 4-pentyloxy-4-(6'-hydroxyhexyl)-trans-stilbene, which was esterified with methacryloyl choride affording one of the two methacrylate monomers. A similar route was followed to two substituted styrenes. All monomers were polymerized by heating in benzene using azo-bis-isobutyronitrile as the free radical initiator. The polymers were purified by repeated extraction and precipitation and characterized by NMR, UV-Vis, GPC, and DSC measurements (Supported by the NSF EPSCoR program).
3:00 SYNTHESIS OF LIQUID CRYSTAL POLYMERS WITH CINNAMATE ESTER CHROMOPHORES
David Creed, Charles E. Hoyle, and Allen Spencer*, University of Southern Mississippi, Hattiesburg, MS 39406-5043
Liquid crystalline (LC) materials combine one or two dimensional order with the ability to flow. The synthesis and characterization of side-chain thermotropic LC polymers containing a UV-sensitive cinnamate ester chromophore are described. The phase behavior, polymerization, and photopolymerization of compounds such as 1 will be described.
![]()
3:15 QUENCHING OF A LIQUID CRYSTALLINE POLY(ARYL CINNAMATE)
David Creed, Charles E. Hoyle, and Alline M. Peeler*, University of Southern Mississippi, Hattiesburg, MS 39406-5043
Films of a main-chain liquid crystalline polymer containing aryl cinnamate chromophores can be prepared in four different phases; the 'as cast' film phase (A), a nematic phase (N), the isotropic phase (I), and a glassy nematic phase (NG) formed by cooling either the I or N phases. Extensive chromophore aggregation occurs in the N and NG phases. In the fluid nematic phase, irradiation induced hyperchromism is observed that is attributed to disruption of chromophore aggregates as 2+2 cycloadducts begin to form. In the A and NG phases, UV-VIS spectroscopic changes indicate that 2+2 cycloaddition and photo-Fries rearrangement are the only photochemical reactions taking place. We have synthesized diene and triene quenchers, 1, for mechanistic studies of the photochemistry of poly (aryl cinnamate) main chain liquid crystalline polymers. The quenchers are designed to be compatible with the LC phases of the polymer and will be used to explore the role of triplet states in the photochemistry of the polymer.